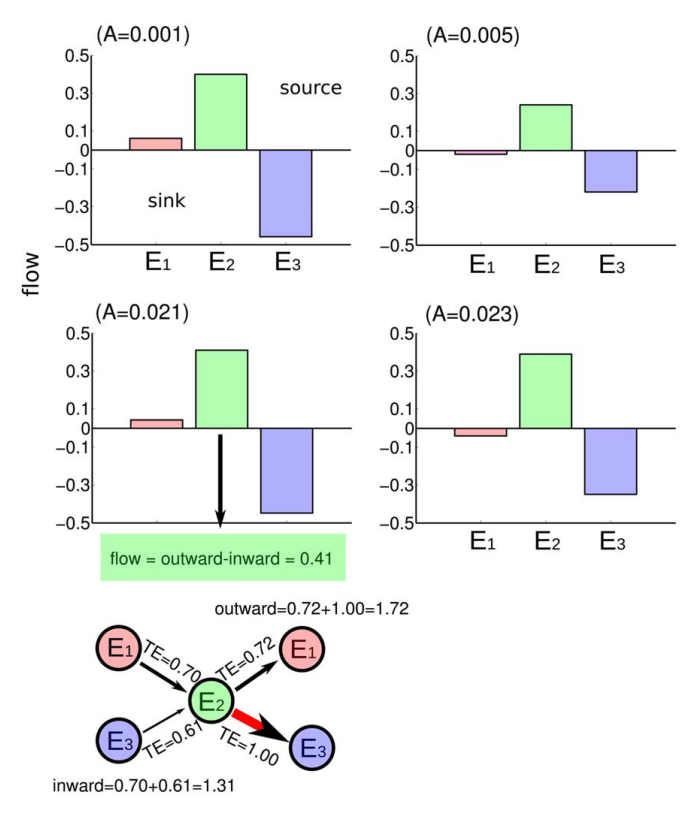
Ildefonso M. De la Fuente and Jesus M. Cortes. Quantitative analysis of the effective inter-enzyme connectivity in glycolysis. PLoS ONE 7:e30162, 2012 [pdf]
Yeast glycolysis is considered the prototype of dissipative biochemical oscillator. In cellular conditions, under sinusoidal source of glucose, the activity of enzymes can display either periodic, quasi-periodic or chaotic behavior. By means of a system of non-linear differential equations with delay we have obtained different time series of catalytic activity in yeast glycolysis. The time series correspond to a quasi-periodic route to chaos in agreement with experimental conditions. Using the non-linear analysis technique of Transfer Entropy we have quantified the effective connectivity for the activity of different enzymes. The results of this analysis are three-fold: first, the information flow between the enzymes is not always the same but varies depending on the substrate fluxes and the dynamic characteristics emerging in the system; second, trough the quasi-periodic route to chaos the pattern of effective connectivity seems to preserve and be an invariant; and finally, similar to what was previously thought, our analysis reveals in a quantitative manner that the enzyme phosphofructokinase is the key-core of glycolysis, behaving for all conditions as the main source of causality flow.